How To Calculate Moles With Avogadro's Number
Chemical ppt powerpoint presentation relationships classification reactions mass formula Mass molar mole moles avogadro atoms molecules grams constant calculate number between particles convert examples converting onlinemathlearning solutions Lab engaging station activities energy ecosystems potential gravitational kinetic mole number adaptations behavioral physical avogadro stations teachsciencewithfergy
Mole Concept and Avogadro Number - YouTube
As chemistry: the mole and the avogadro constant by ja2010 Mole concept and avogadro number Calculation of one mole
Calculate avogadro mole calculation
Avogadro number mole mass moles chapter ppt powerpoint presentation equations atoms slideserve molAvogadro number moles chapter formula na ppt powerpoint presentation What is the relationship between a mole and avogadro's numberHow to calculate number of particles| avogadro’s constant| mole.
Random stuff: stoichiometry note #1: the mole and the avogadro constantAvogadro mass number molar mole concept Mole relationship number between avogadro mass particles avogadros other interconversion intoAvogadro constant using moles question.

Chemical calculations
Calculating moles using avogadro's numberMoles atoms molecules avogadro calculating What is the relationship between a mole and avogadro's numberAvogadro’s number.
Mole avogadro number mass ppt powerpoint presentation molecule reactions classification chemical relationships1. density mass volume 3. avogadro's number moles number of How to work out molesAvogadro atoms converting moles.

Moles atoms avogadro occurred error
Avogadro constant moleParticles number avogadro constant calculate mole The moleAvogadro number moles using calculating.
Avogadro moleUsing moles and avogadro's constant question markscheme. Avogadro number mass mole molecules between relation chemistry atomsCalculations mole chemical moles calculate equation involving.

As chemistry: the mole and the avogadro constant
Moles to atomsMole, avogadro constant & molar mass (solutions, examples, videos) Number mole relationship avogadro between avogadros moles concept molarity chemistry solute mass particles aplustopper into litre solution choose board otherAvogadro constant mole chemistry.
The mole and avogadro’s number – 7 engaging lab stationsNumber avogadro concept What is the relationship between a mole and avogadro's numberAvogadro moles number using calculations mole chemistry.

Mass moles volume density avogadro number chemistry used which
Mole number relationship between avogadro formula avogadros empirical chAvogadro constant mole note stoichiometry molecules moles converting next .
.

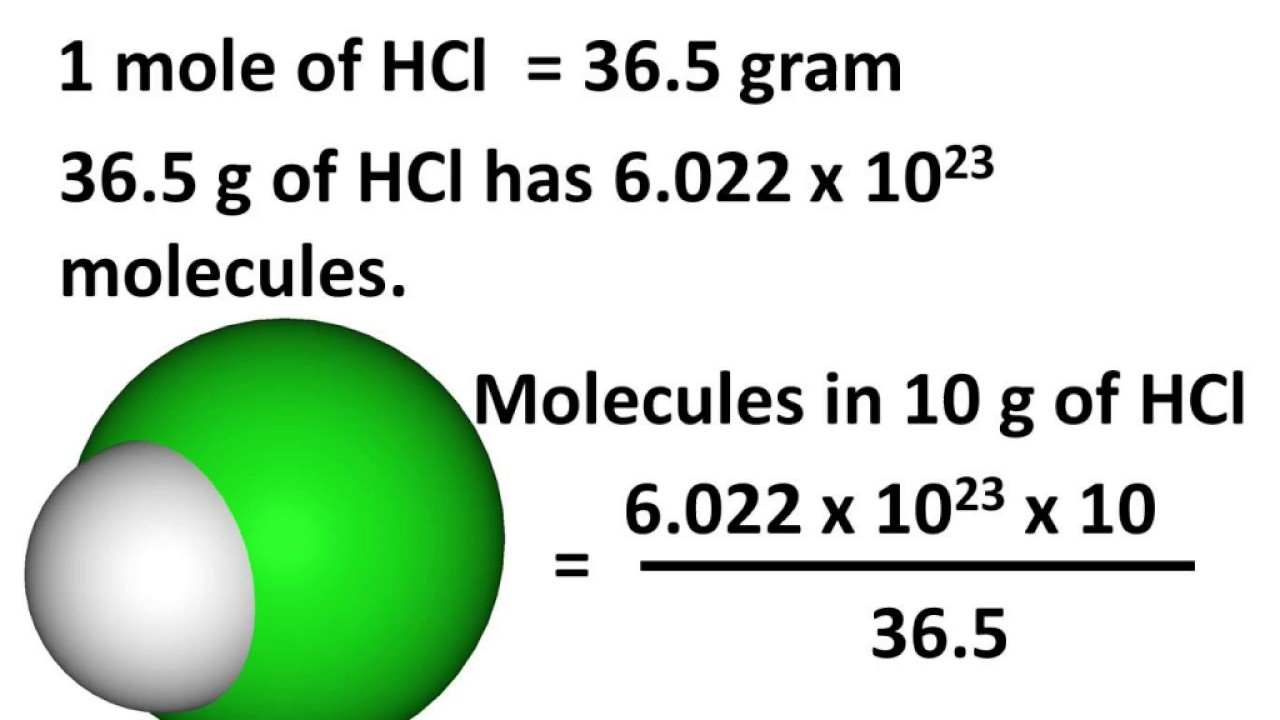
Chemistry - Relation between Mole, Avogadro number and Mass - Atoms and

Mole Concept and Avogadro Number - YouTube

Mole, Avogadro Constant & Molar Mass (solutions, examples, videos)

What is the Relationship between a Mole and Avogadro's number - A Plus

PPT - Chapter 19 PowerPoint Presentation, free download - ID:3073897

What is the Relationship between a Mole and Avogadro's number - A Plus

AS Chemistry: The Mole and The Avogadro Constant by ja2010 | Teaching